Release date: 2022-05-27 clicks: 4643
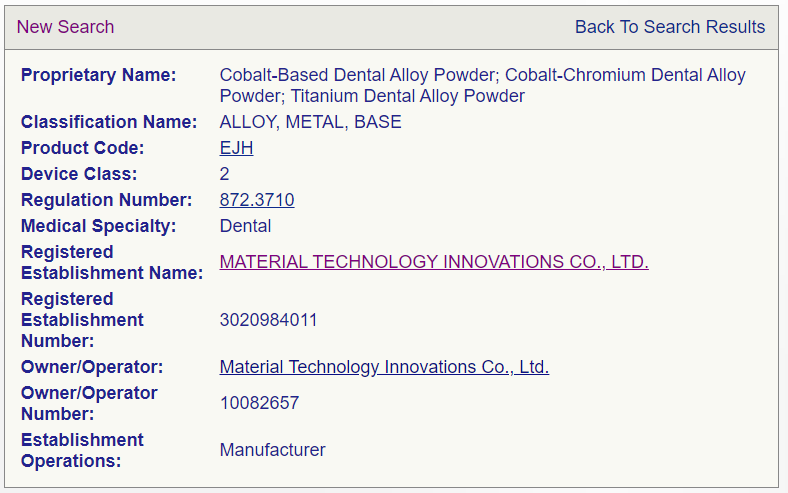
Congratulations to MTI for obtaining ISO 13485:2016&Dental alloy powder FDA market entry license!
Material Technology Innovations Co., Ltd. (abbreviation below: MTI) have passed the ISO 13485:2016 system inspection and obtained the relevant FDA market entry license on 3rd May, 2022.
For the following design and manufacture of Cobalt-based Dental Alloy Powder, Cobalt-chromium Dental Alloy Powder and Titanium Dental Alloy Powder, the products can be used for 3D printing of dental prostheses, crowns, bridges,inlays, post cores,removable partial denture brackets and clasp for fixing dentures .
ISO system certification and FDA will help the company to continues improve. At the same time, this marks the products of MTI have reached the international advanced level in terms of quality and safety performance, which would accelerating the pace of opening the overseas markets.